Ultrafine powders refer to materials with particle sizes ranging from micrometers to nanometers. According to the consensus in China’s mineral processing industry, ultrafine powders are those with 100% of particles smaller than 30 μm. Nano materials are widely used. They have unique properties that traditional materials lack—such as small size effects, macroscopic quantum tunneling effects, and surface effects.

However, nanomaterials have a high specific surface area, strong activity, and are extremely unstable. They tend to agglomerate easily, losing their original properties. This decreases their value and complicates both preparation and storage. Therefore, agglomeration is a key technical issue restricting the development of nanomaterials.
Agglomeration of Ultrafine Powders
Agglomeration refers to the process where primary powder particles connect during preparation, separation, processing, or storage, forming larger clusters. Currently, three main causes of ultrafine powder agglomeration are as follows:
Intermolecular Forces
When mineral particles change into ultrafine scale, the distance between them becomes very small. Van der Waals forces then exceed the gravitational force of the particles themselves, leading to attraction and agglomeration. Hydrogen bonding, adsorbed moisture bridges, and other chemical bonds on the particle surfaces also promote adhesion and clustering.
Electrostatic Forces
During ultrafine grinding, impact and friction cause particles to accumulate positive or negative charges on their surfaces. Some protrusions on a particle may be positively charged while others are negatively charged. These unstable, charged particles attract each other—especially at sharp points—resulting in agglomeration. The main driving force in this process is electrostatic attraction.
Adhesion in Air
When the relative humidity of the air exceeds 65%, water vapor condenses on and between particles. This creates liquid bridges that significantly enhance agglomeration.
Additionally, during crushing, mineral materials absorb mechanical or thermal energy. This gives newly formed ultrafine particles high surface energy, making them unstable. To reduce this energy, particles naturally move closer and agglomerate.
Nanomaterial agglomeration include soft type and hard type. Soft agglomeration is caused by van der Waals and intermolecular forces and is relatively easy to reverse. Hard agglomeration is more complex, with five main theories proposed: capillary adsorption, hydrogen bonding, crystal bridging, chemical bonding, and surface atom diffusion. However, no unified explanation has yet been accepted.
Despite these challenges, people have conducted extensive research to develop dispersion technologies for preventing agglomeration.
Dispersion of Ultrafine Powders
Dispersion techniques mainly focus on two states: dispersion in gas-phase media and in liquid-phase media.
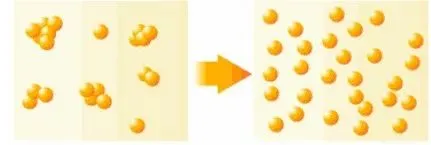
1. Mechanical Dispersion
This method uses external mechanical energy—such as shear or impact—to disperse nanoparticles in a medium. Common techniques include grinding, ball milling, vibration milling, colloid milling, air jet milling, and mechanical stirring.
However, once particles leave the turbulent field created by stirring, they may re-agglomerate. Therefore, combining mechanical dispersion with chemical dispersants often produces better results.
2. Chemical Dispersion
Widely used in industry, this method involves adding electrolytes, surfactants, or polymer dispersants to a suspension of ultrafine powders. These agents adsorb on particle surfaces, alter surface properties, and improve compatibility with the liquid phase, leading to better dispersion.
Common dispersants include surfactants, low-molecular inorganic salts, polymer dispersants, and coupling agents. Polymer dispersants—especially polyelectrolytes—are among the most widely used and effective.
3. Ultrasonic Dispersion
Ultrasonic dispersion involves placing a suspension in an ultrasonic field and applying suitable frequency and duration to achieve effective particle separation.
Ultrasound generates localized high temperatures, high pressure, strong shockwaves, and microjets. These forces weaken particle interactions, aiding dispersion. However, overheating must be avoided—excess thermal and mechanical energy may increase collision frequency and worsen agglomeration.
Dispersion in Gas Phase
1. Dry Dispersion
In humid air, liquid bridges form between particles and cause agglomeration. Drying solid materials involves two basic steps: heating to vaporize moisture, and allowing vapor to diffuse into the gas phase. Eliminating or breaking liquid bridges is essential for maintaining good dispersion.
Most powder production processes include thermal drying as a pretreatment step.
2. Mechanical Dispersion
This method uses mechanical forces—such as shear and compressive stress—greater than particle adhesion forces to break up clusters. Common sources include high-speed rotating impellers, discs, or high-velocity air jets creating intense turbulence.
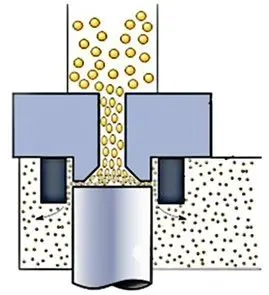
Mechanical dispersion is relatively easy to implement. However, because it is a forced process, once particles leave the disperser, they may re-agglomerate. It may also damage fragile particles and lead to reduced effectiveness as equipment wears.
3. Electrostatic Dispersion
Particles of the same material with identical charges repel each other due to electrostatic force. This principle is used for dispersion—if particles can be fully charged.
Charging methods include contact, induction, and corona charging. Among these, corona charging is the most effective. It creates an ion curtain through corona discharge, which charges particles uniformly. The resulting repulsive forces help maintain dispersion.
Conclusion
There are many methods for modifying ultrafine powders, differing greatly from the main approaches discussed above. We need to both optimize modification processes based on in-depth studies and develop composite techniques that serve multiple functions. In short, progress in ultrafine powder technology requires collaboration across the entire industry—from research institutions to manufacturers—and continuous innovation.
Choose Epic Powder for efficient, energy-saving, and environmentally friendly powder processing solutions!
